COVIND is a global registry of COVID-19 individual patient data. It accelerates pooling of high-quality patient data, while ensuring the strongest data protection standards.
Why COVIND?
A lot of clinical studies about COVID-19 are being published. However, most of them only release aggregated cohort data, without individual patient data. This makes secondary data analysis much more difficult. For example:
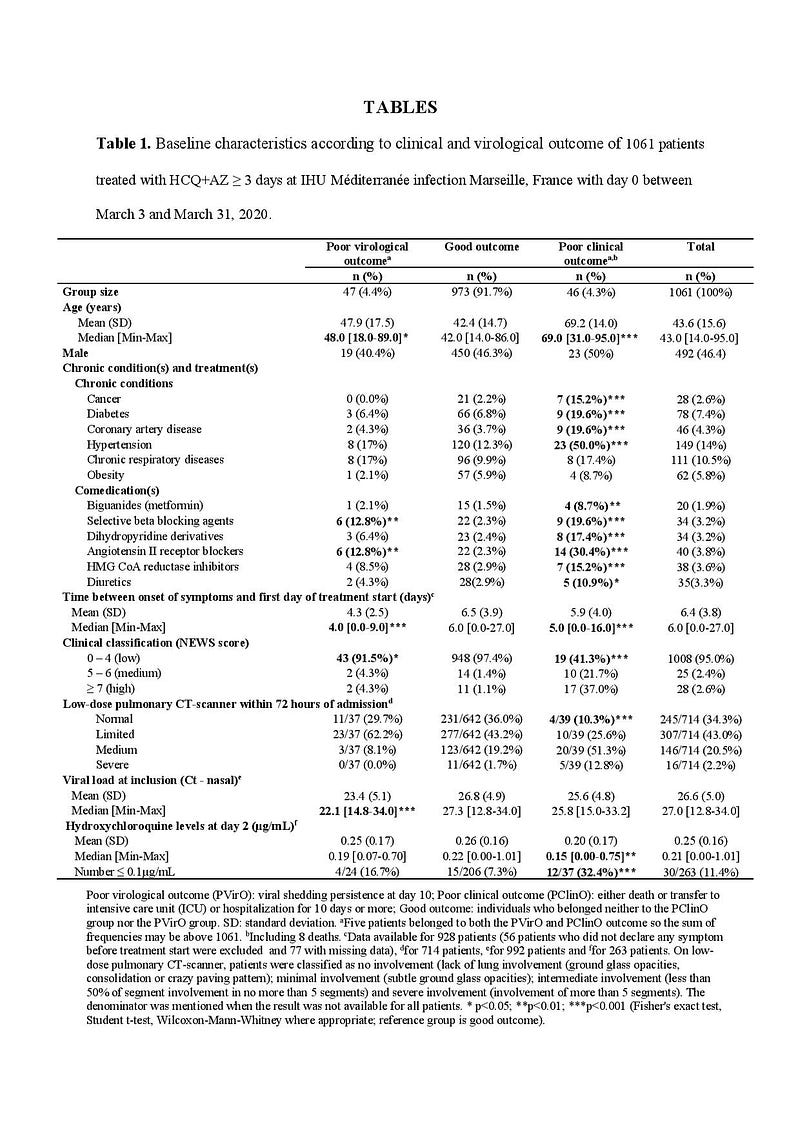
Clinical outcomes heavily depend on individual patient characteristics, which are lost by data aggregation.
For example, these 2 datasets with 2 patients are the same at the aggregated level, but they can still give different clinical outcomes:

On the other hand, COVIND database will comprise individual pseudonymized data. It will look like:
We also plan to add CT scans and other relevant information from electronic health records.
COVIND use-cases
With COVIND database, we plan to better evaluate therapies, such as:
- Chloroquine
- Azithromycin
- Tocilizumab
- Artemisia
- Remdesivir…
We also plan to evaluate interventions such as:
- Extra-Corporeal Membrane Oxygenation
- Invasive Ventilation
- Helmet Ventilation
- High-Flow Nasal Cannula…
These projects will rely on the latest AI and machine learning technologies, as outlined in this article.
COVIND is patient-centered
In addition to their revocable and explicit consent, patients will be able to monitor the impact of their data donation on scientific findings, on a platform suitable for a general audience. This will help them understand research purposes. With this pandemic, appetite for research in infectious diseases is growing among the public. Moreover, this platform will facilitate patient long-term follow-up.
COVIND is GDPR-compliant
Data protection is guaranteed by the European GDPR, one of the strictest privacy regulations in the world. We can also comply with other local regulations upon request.

Membership benefits for hospitals
- Accelerate research by accessing the consortium network
- Upgrade hospital IT infrastructure, with state-of-the-art technologies in artificial intelligence and big data
To join the consortium as a doctor, sponsor or partner, drop a message to Mostapha Benhenda (mostapha@melwy.com), or fill the form.
Members
- Assiut, Egypt
Aliae Hussein, MD
Professor, Department of Chest Diseases, Faculty of Medicine, Assiut University
- Brescia, Italy
Elena Torri, MD
BresciaMEd - Ambulatorio Medico ecografie e visite urgenza
- Cambridge, Massachusetts, United States of America
Christopher Barrett, MD
Affiliate MIT Researcher
Beth Israel Deaconess Medical Center
Harvard Medical School
Michael B. Yaffe, MD
Director of the Yaffe Lab
Professor of Biology and Biological Engineering, Koch Institute for Integrative Cancer Research, Center for Precision Cancer Medicine at MIT
Beth Israel Deaconess Medical Center
- Cremona and Trieste, Italy
Daniele Generali, MD
Direttore di U.O. Multidisciplinare di Patologia Mammaria e Ricerca Traslazionale, Azienda Socio-Sanitaria Territoriale di Cremona
Professore Associato in Oncologia Medica
Dipartimento Universitario Clinico di Scienze Mediche, Chirurgiche e della Salute, Università degli Studi di Trieste
- Denver, Colorado, United States of America
Ernest E. Moore, MD
Ernest E. Moore Shock Trauma Center at Denver Health
Hunter Moore, MD
Division of Transplant Surgery, Department of Surgery, University of Colorado
- Lahore, Pakistan
Ahmad Imran, MBBS
Shaikh Zayed Hospital
Qurrat ul Ain Iqbal, MBBS
Shaikh Zayed Hospital
- London, United Kingdom
Emmanuel Ako, MD
Department of Cardiology at Chelsea and Westminster NHS hospital
- Moscow, Russia
Altana Mikhakhanova, MD
NPO Petrovax Pharm LLC, a full-cycle biopharmaceutical company
- Paris, France
Mostapha Benhenda, PhD
Data scientist, Melwy
- Rome, Italy
Danilo Buonsenso, MD
U.O.C. di Pediatria, Fondazione Policlinico Universitario “A. Gemelli”
- Tehran, Iran
Seyed Sina Naghibi Irvani, MD, MPH, MBA
Shahid Beheshti University of Medical Sciences
- Tübingen, Germany
Diane Egger-Adam, MD, Project Manager
Institute of Tropical Medicine, University of Tübingen