Cancer drug development is very risky. The pipeline suffers from a lot of attrition: only 3.4% of human-tested oncology drugs reach the market. It means 96.6% fail. To reduce failure, one idea is to feed clinical data back into early drug development, from bedside to bench.
That’s what I did in my last paper. I fed back a predictive model, trained on 73 human cancer studies, into the pre-clinical development of a CAR T cell therapy for solid tumors:
What is CAR T cell therapy?
CAR T cells are an emerging therapy tweaking immune T cells with Chimeric Antigen Receptors (CAR), so that they become fast, furious and focused on tumors:
CAR T therapies are already approved in few liquid cancers, since 2017. They are marketed as Kymriah and Yescarta, with furious prices, up to $475,000 per patient.
On the other hand, CAR T therapies still fail in solid cancers, partly because once inside tumors, CAR T cells get exhausted too quickly. CAR T cells are sprinters, but fighting cancer is a marathon. By being too fast & too furious, they burn-out.

“Exhaustion-resistant” JUN CAR T cells
To overcome exhaustion, Crystal Mackall’s lab, at Stanford Center for Cancer Cell Therapy, engineered CAR T cells to over-express c-Jun protein, coded by the JUN gene. They published a paper.
CAR T cells become stronger indeed, but at the end, improvement is modest: they were only able to extend survival of cancerous mice by 20 days:
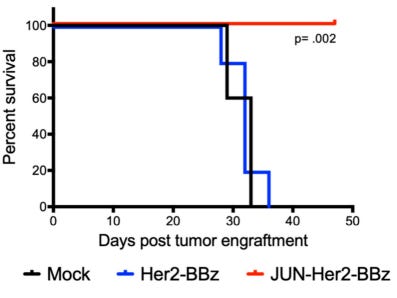
After 50 days, the fate of surviving mice is unknown. I hope the final cell therapy will guarantee longer survival, because with an expected price of half-Million dollar per therapy, the cost of 1 year of life extension will be $8 Million. Market regulators around the world often reject cancer drugs that are much cheaper.
To monitor CAR T cells health and activity, Mackall’s team relies on several single-gene biomarkers of exhaustion. For example, they measure expression levels of PDCD1, TIGIT and TOX genes. With these uni-dimensional indicators, everything seems fine indeed. However, if we consider advanced genome-wide biomarkers, designed for tumor micro-environment monitoring, the picture is a bit different.
Clinical data feedback with TIDE dysfunction biomarker
I applied the dysfunction biomarker TID (Tumor Immune Dysfunction), a component of TIDE (Tumor Immune Dysfunction and Exclusion), developed by Xiaole Shirley Liu’s lab, at Harvard Cancer Center. TID is a predictive model trained on 73 human cancer studies, aggregating tens of thousands of samples. TID combines a lot of late-stage clinical data.

TIDE was initially designed with another immunotherapy in mind: immune checkpoint inhibitors. These immunotherapies also often fail because of T cells exhaustion. However, TIDE relies on general survival data, so it makes sense to re-purpose this predictive model for CAR T cells therapies.
Learn more about TIDE in my quick video (< 1 minute):
And don’t miss this in-depth video by TIDE original author Xiaole Shirley Liu (~ 10 minutes):
“Exhaustion-resistant” JUN CAR T cells vs. TIDE dysfunction biomarker
When we confront “exhaustion-resistant” JUN CAR T cells, and TIDE dysfunction biomarker (TID), what happens?
Good news: TIDE dysfunction biomarker, TID, confirms that on average, JUN CAR T cells are less dysfunctional (their difference with non-JUN cells is negative):
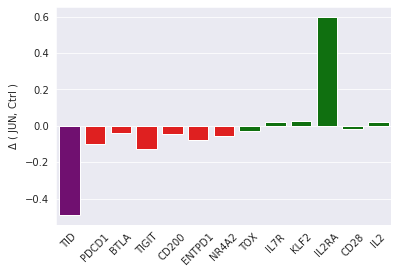
Bad news: TID also reveals that JUN CAR T cells are heterogeneous. This was not apparent with single-gene biomarkers:
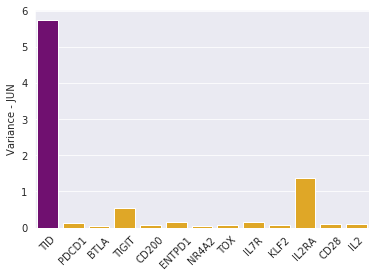
TID cell variance in purple, single-gene variances in yellow.
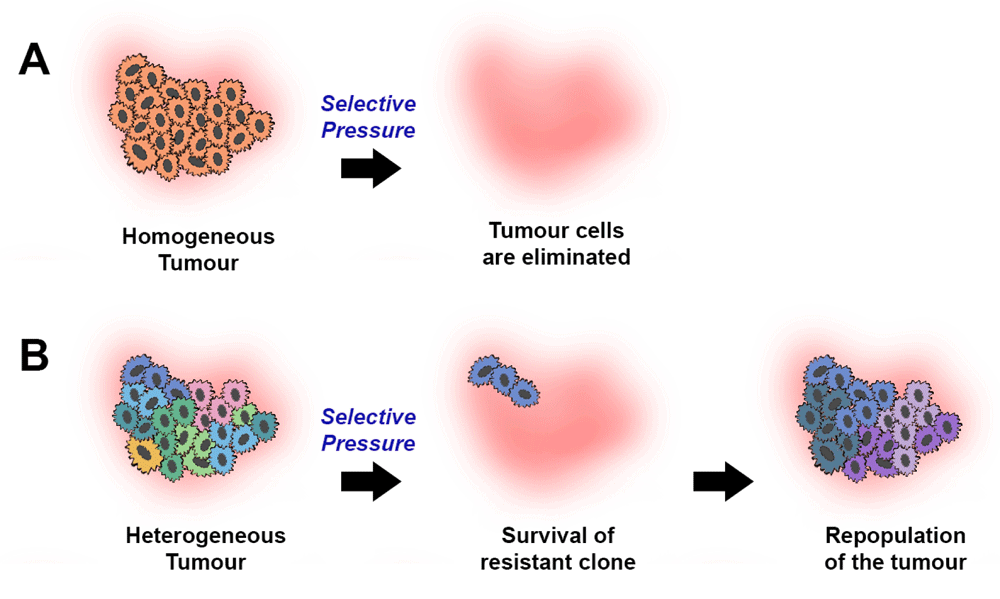
Heterogeneity is very bad in cancer: it brings opportunities for tumor resistance.
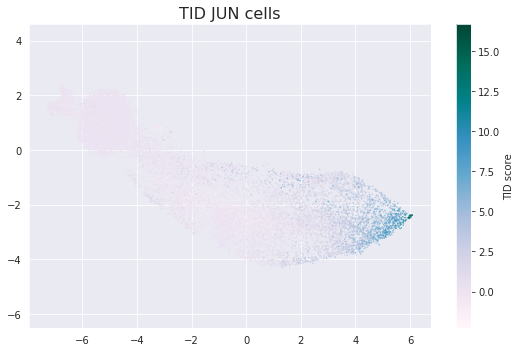
For further investigation, one possibility is to look at low-dysfunctional JUN CAR T cells in detail. They cluster at the tip of 2-D cell projection, and that’s remarkable:
Conclusion
If I was a decision-maker, I would request additional experiments, data and analysis. For example, if I was CEO of Lyell Immunopharma (the Stanford spin-off), or a biotech Venture Capitalist, I would ask for de-risking JUN CAR T cells further, before investing in a clinical trial. It’s better to nail down the pre-clinical phase, before moving forward.
Of course, there are several caveats: tumor resistance, if any, didn’t have enough time to show up within a limited 50-days mouse experiment. Moreover, Mackall’s team did not publish tumor cells data, only CAR T cells data. I am supposing heterogeneity is related in both cell types. Finally, TIDE biomarker also has uncertainty (see my paper for details).
Anyway, that’s an exciting project, and if you want to play with the code and data, it’s available upon request at Melwy.com. I prepared a hands-on tutorial, with a lot of exercises.
I also recommend you to join other fascinating projects about Cancer & Data Science, I organize Telegram and Slack groups.